Search Results
6/23/2025, 12:09:06 AM
>>508385370
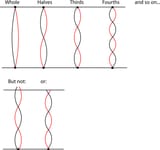
Also some American university online course (MIT) professor about solid state chemistry told in the video lecture that electrons act as standing waves. They need specific “trajectories” to exist with non destructive interference, and each atom has specific number of electrons in “quantum” s p d f trajectories for this reason. Also, when atoms form bonds to molecules, the standing wave electrons now oscillate around the whole molecule and give incalculable properties many times. Explaining full molecule effects for proteins etc computationally is a joke. That’s why concepts as neural networks to imitate life are ridiculous. I liked the standing wave electron explanation
Also some American university online course (MIT) professor about solid state chemistry told in the video lecture that electrons act as standing waves. They need specific “trajectories” to exist with non destructive interference, and each atom has specific number of electrons in “quantum” s p d f trajectories for this reason. Also, when atoms form bonds to molecules, the standing wave electrons now oscillate around the whole molecule and give incalculable properties many times. Explaining full molecule effects for proteins etc computationally is a joke. That’s why concepts as neural networks to imitate life are ridiculous. I liked the standing wave electron explanation
Page 1