Search Results
7/10/2025, 3:04:20 PM
ALERT: BioNTech just submitted new RMP (Risk Management Plan) version to
@EMA_NEWS
in order to:
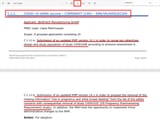
1-Revise study C4591048 (mRNA Vaccine in healthy children)
2-Remove missing information "Use in pregnancy and while breast feeding" from the list of safety concerns with removal of the study C4591022=Teratology Trial C459102 (study of Congenital Abnormalities and Abnormal Formations) which is not yet completed and no results yet published.
Why would Pfizer BioNTech want to remove the warning about pregnancy safety concerns and references to the ongoing study of congenital abnormalities and abnormal formations, even though the study is still incomplete and lacks available results??
The end of this Section 5.22 states "Action: For Adoption," so it wouldn’t be surprising if
@EMA_NEWS
proceeds to approve the changes, deeming them safe without requiring C4591022 study completion or results!
@IamBrookJackson
Source:
https://ema.europa.eu/en/documents/agenda/agenda-prac-meeting-7-10-july-2025_en.pdf
https://catalogues.ema.europa.eu/sites/default/files/document_files/C4591022_PROTOCOL%20AMENDMENT%203_V4_09MAY2022.pdf
https://catalogues.ema.europa.eu/node/3169/administrative-details
https://x.com/VaccineMole/status/1942745612774171083
@EMA_NEWS
in order to:
1-Revise study C4591048 (mRNA Vaccine in healthy children)
2-Remove missing information "Use in pregnancy and while breast feeding" from the list of safety concerns with removal of the study C4591022=Teratology Trial C459102 (study of Congenital Abnormalities and Abnormal Formations) which is not yet completed and no results yet published.
Why would Pfizer BioNTech want to remove the warning about pregnancy safety concerns and references to the ongoing study of congenital abnormalities and abnormal formations, even though the study is still incomplete and lacks available results??
The end of this Section 5.22 states "Action: For Adoption," so it wouldn’t be surprising if
@EMA_NEWS
proceeds to approve the changes, deeming them safe without requiring C4591022 study completion or results!
@IamBrookJackson
Source:
https://ema.europa.eu/en/documents/agenda/agenda-prac-meeting-7-10-july-2025_en.pdf
https://catalogues.ema.europa.eu/sites/default/files/document_files/C4591022_PROTOCOL%20AMENDMENT%203_V4_09MAY2022.pdf
https://catalogues.ema.europa.eu/node/3169/administrative-details
https://x.com/VaccineMole/status/1942745612774171083
Page 1