Search Results
7/13/2025, 7:35:50 PM
>>280533390
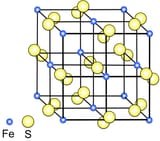
Iron atoms are more stable with less electrons, sulphur atoms are more stable with more electrons so sulfur takes some electrons from iron but that makes sulfur negative and iron positive so the two attract each other and repel other atoms with similar charge. (positive iron repels positive iron, negative sulfur repels negative sulfur, positive iron attracts negative sulfur) so they pile up like a bunch of magnets.
Iron atoms are more stable with less electrons, sulphur atoms are more stable with more electrons so sulfur takes some electrons from iron but that makes sulfur negative and iron positive so the two attract each other and repel other atoms with similar charge. (positive iron repels positive iron, negative sulfur repels negative sulfur, positive iron attracts negative sulfur) so they pile up like a bunch of magnets.
Page 1