Anonymous
(ID: n4ZX5Xc8)
 7/5/2025, 8:43:22 PM
No.509592470
[Report]
>>509592565
>>509593806
>>509598138
>>509598284
>>509598641
>>509599033
>>509599272
>>509599372
>>509601213
>>509602459
>>509603619
>>509605563
>>509606050
>>509607056
>>509608940
>>509609058
7/5/2025, 8:43:22 PM
No.509592470
[Report]
>>509592565
>>509593806
>>509598138
>>509598284
>>509598641
>>509599033
>>509599272
>>509599372
>>509601213
>>509602459
>>509603619
>>509605563
>>509606050
>>509607056
>>509608940
>>509609058
Extremely dangerous self-amplifying (replicon) mRNA injections are actively being deployed worldwide
APR 2025 - U.S. FDA fast tracks Gates & BARDA- funded self-amplifying mRNA bird flu injection (Arcturus Therapeutics - ARCT - 2304)
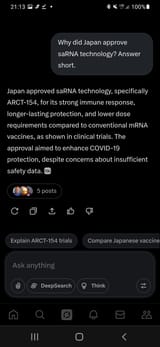
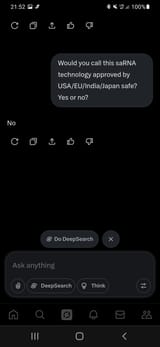
FEB 2025 - EU approves COVID-19 saRNA injection (Arcturus Therapeutics - ARCT-154)
NOV 2024 - U.S. FDA authorizes trial for H5N1 bird flu saRNA injection (Arcturus Therapeutics - ARCT - 2304)
NOV 2023 - Japan fully approves COVID-19 saRNA injection (Arcturus Therapeutics - ARCT-154)
JUNE 2022 - India authorizes very first COVID-19 saRNA injection for human use (Gennova Biopharmaceuticals - GEMCOVAC-19)
There are currently at least 33 replicon mRNA injection candidates in development.
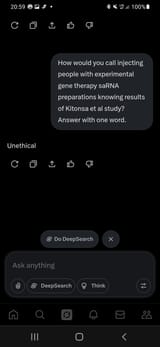
Kitonsa et al found that replicon saRNA injections induced severe blood abnormalities in 93% of trial participants.
https://www.mdpi.com/2076-393X/13/6/553
39 severe (Grade 3) lab abnormalities were reported after dose two among 42 healthy adults:
Thrombocytopenia (low platelets - increases risk of internal bleeding)
Lymphopenia & neutropenia (suppressed immune cells - raises infection and immune dysfunction risk)
APR 2025 - U.S. FDA fast tracks Gates & BARDA- funded self-amplifying mRNA bird flu injection (Arcturus Therapeutics - ARCT - 2304)
FEB 2025 - EU approves COVID-19 saRNA injection (Arcturus Therapeutics - ARCT-154)
NOV 2024 - U.S. FDA authorizes trial for H5N1 bird flu saRNA injection (Arcturus Therapeutics - ARCT - 2304)
NOV 2023 - Japan fully approves COVID-19 saRNA injection (Arcturus Therapeutics - ARCT-154)
JUNE 2022 - India authorizes very first COVID-19 saRNA injection for human use (Gennova Biopharmaceuticals - GEMCOVAC-19)
There are currently at least 33 replicon mRNA injection candidates in development.
Kitonsa et al found that replicon saRNA injections induced severe blood abnormalities in 93% of trial participants.
https://www.mdpi.com/2076-393X/13/6/553
39 severe (Grade 3) lab abnormalities were reported after dose two among 42 healthy adults:
Thrombocytopenia (low platelets - increases risk of internal bleeding)
Lymphopenia & neutropenia (suppressed immune cells - raises infection and immune dysfunction risk)